Moderna Vaccine Efficacy After One Dose : Beyond The First Dose Covid 19 Vaccine Follow Through And Continued Protective Measures Nejm
It was found that up to 14 days after the first dose the effectiveness was 508 percent. 1 and before dose No.
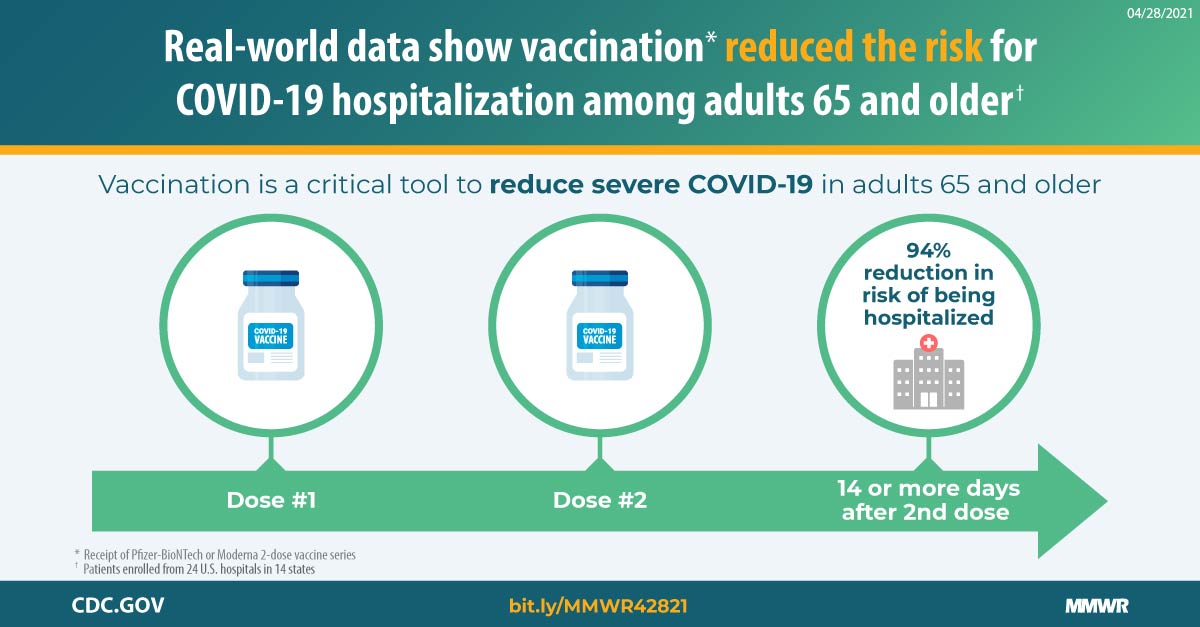
Effectiveness Of Pfizer Biontech And Moderna Vaccines Against Covid 19 Among Hospitalized Adults Aged 65 Years United States January March 2021 Mmwr
A single dose of Pfizers or Modernas Covid-19 vaccine was 80 effective in preventing infections according to a new CDC study.

Moderna vaccine efficacy after one dose. February 19 2021 836am. Moderna vaccine highly effective two weeks after first dose. 51 effectiveness after dose one and 93 after dose two.
How efficacious is the vaccine. There are currently limited data on effectiveness of mRNA COVID-19 vaccines administered beyond this window. Cases of myocarditis and pericarditis in adolescents and young adults have been reported more often after getting the second dose than after the first dose of one of the two mRNA COVID-19 vaccines Pfizer-BioNTech or Moderna.
One dose of Pfizer or Moderna vaccines appears at least 80 effective against symptomatic COVID-19. Does it work against new variants. The Moderna vaccine requires two doses to be fully effective.
However if it is not possible to follow the recommended interval you may schedule the second dose of the Moderna COVID-19 Vaccine for administration up to 6 weeks 42 days after the first dose. 2 on day 21. The samples were collected one week to 31 days after the recipients second vaccine dose.
A study from the CDC reports the first dose of Pfizer or Moderna vaccines is about 80 percent effective. Protection rose to 88 after two doses. The Moderna vaccine has been shown to have an efficacy of approximately 941 per cent in protecting against COVID-19 starting 14 days after the first dose.
695 effective at preventing COVID symptoms between the 1st and 2nd dose. They found that those whod never been infected by SARS-CoV-2 developed antibodies at low levels within 9 to 12 days of receiving their first dose of vaccine. Protection after one dose based on analysis of late-stage trial data.
The second dose pushes effectiveness up to 90. Even ONE dose of Modernas COVID-19 vaccine is effective. According to a document the company submitted to the FDA the Moderna vaccine can provide 802 protection after one dose compared to 956 after the second in people aged 18 to 65.
At least 80 and probably better than 90 after a single dose 28 days later Unclear after 28 days because it hadnt been tested Moderna vaccine. Two doses are better than one the CDC said. Those protection levels for delta are lower than what they found for the older alpha variant.
These reports are rare and the known and potential benefits of COVID-19 vaccination outweigh the known and potential risks including the possible risk of. Healthcare workers who received a single shot were 95 less likely to contract the. Single doses of the Pfizer and Moderna vaccines are more than 92 percent effective in preventing COVID-19.
The range was between 52 and 97. The same Canadian study that found the Pfizer-BioNTech vaccine to be 87 percent effective suggested that the Moderna vaccine was 72 percent. A study published in JAMA involving healthcare workers found that the first dose of Modernas NSDQMRNA COVID-19 vaccine was highly effective at reducing the risk of COVID-19 infections.
Using the data from the published study of the Pfizer vaccine Public Health England determined that vaccine efficacy was 89 for 15 to 21 days after dose No. To find out the researchers enlisted the help of 109 people whod received their first dose of mRNA vaccines made by either Pfizer or Moderna. For days 15 to 28 or up to the first week after the second dose protection from the first dose was estimated at 91.
Another real-world study of adults ages 70 and older conducted by Public Health England in early 2021 determined that a single dose of the Pfizer vaccine was 61 effective at preventing. After that it was about 921 percent. For AstraZeneca one-shot efficacy is 70.
Although subjects were not randomly assigned in the study the vaccine that was administered was dictated by local availability at the time of vaccination with 79 receiving Pfizer and 88 receiving Moderna.

Public Health Impact Of Delaying Second Dose Of Bnt162b2 Or Mrna 1273 Covid 19 Vaccine Simulation Agent Based Modeling Study The Bmj
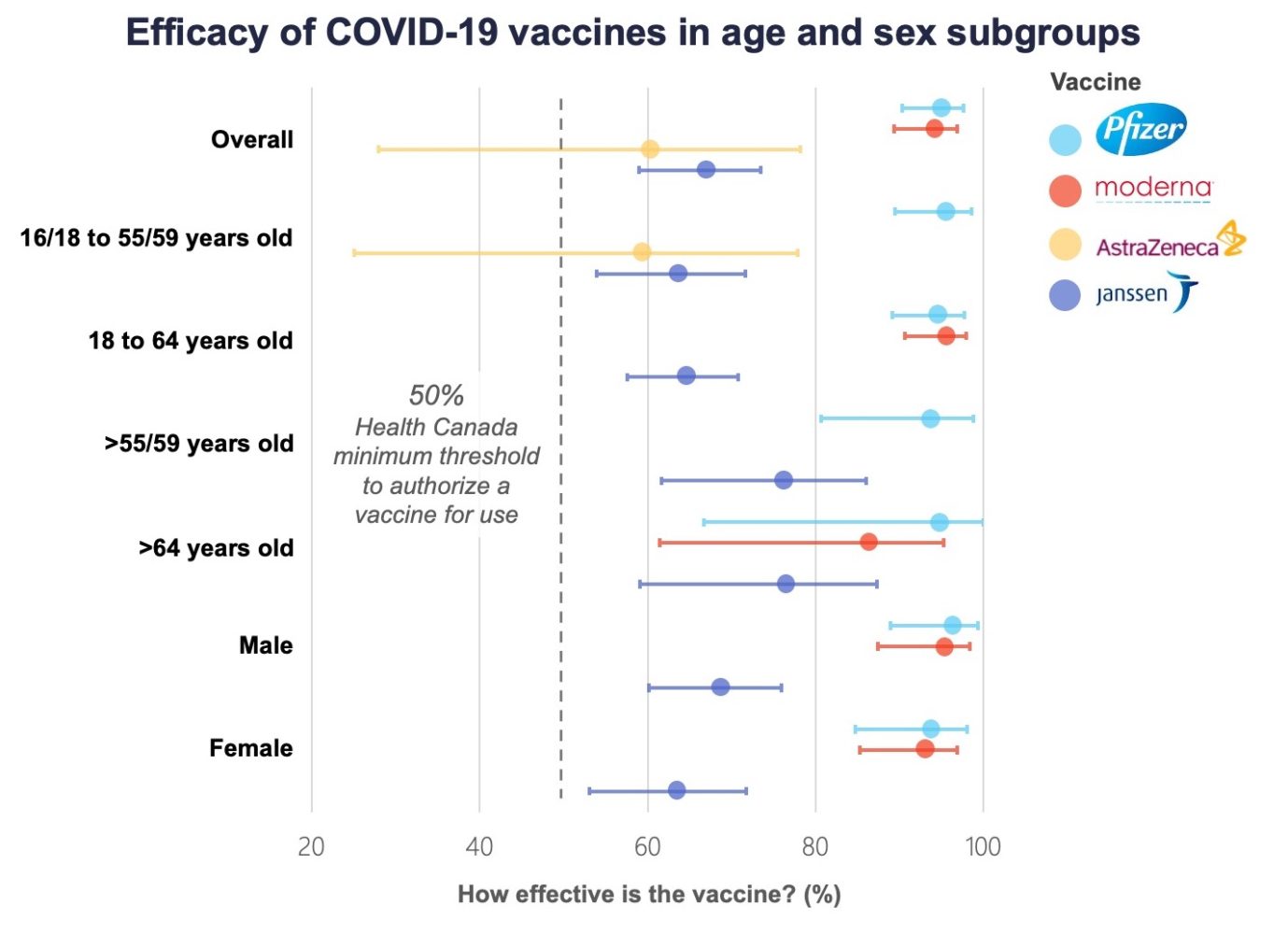
Comparing Vaccines Efficacy Safety And Side Effects Healthy Debate
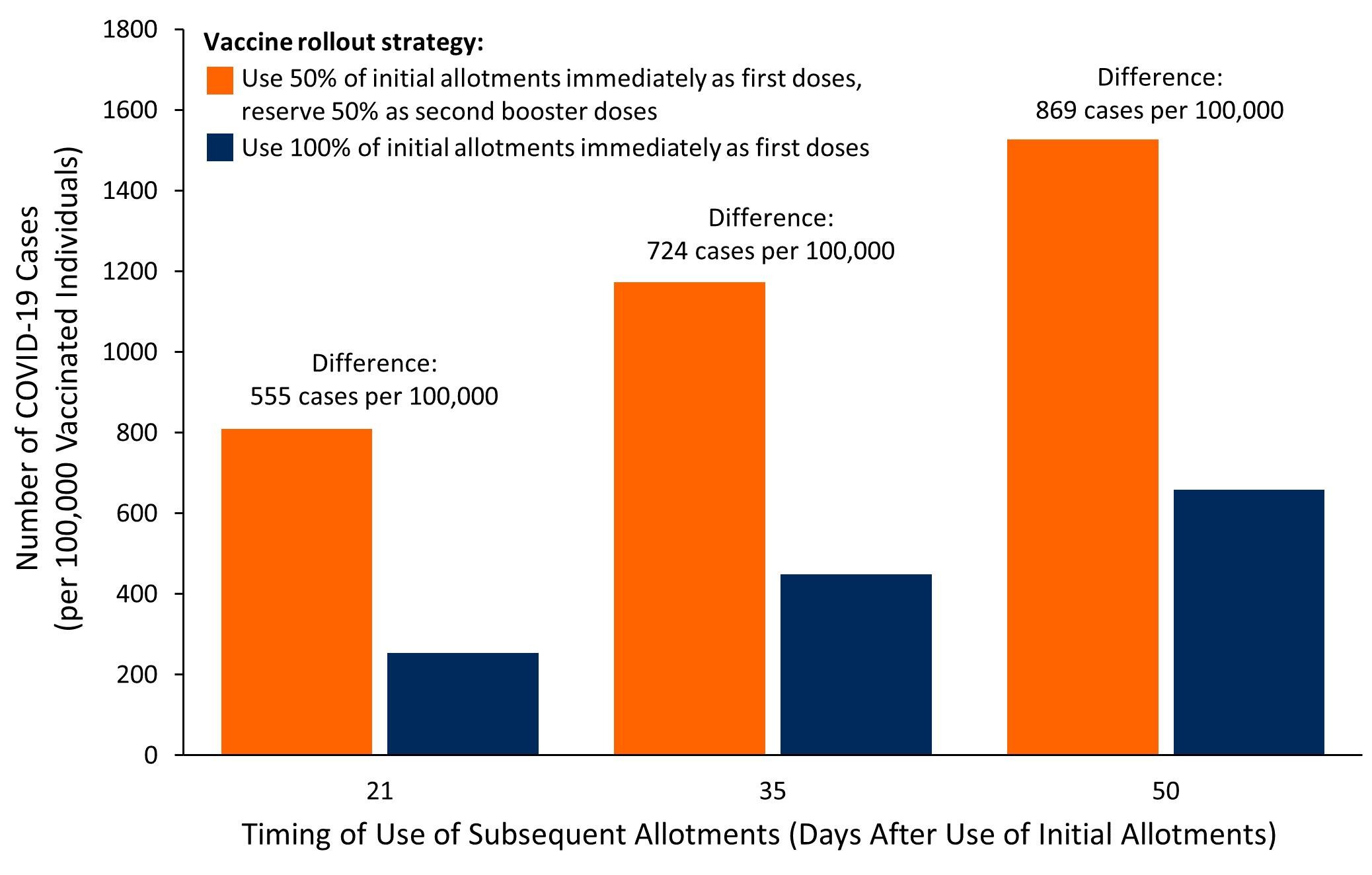
Rollout Strategy For The Pfizer Biontech Covid 19 Vaccine In Ontario Ontario Covid 19 Science Advisory Table
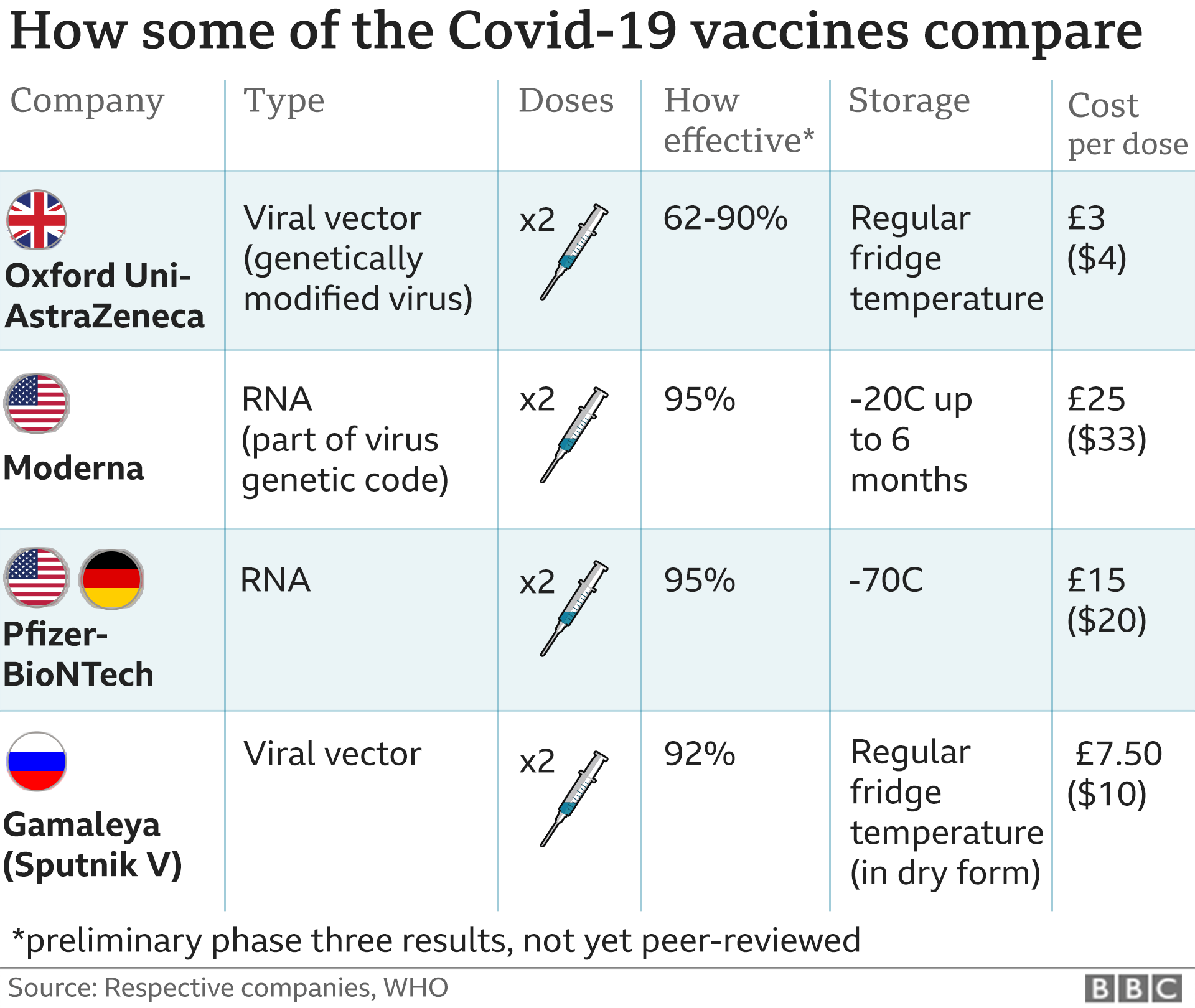
Covid Vaccine Moderna Seeks Approval In Us And Europe Bbc News
Chart How Well Moderna Vaccine Prevents Covid 19 Infections

Mrna 1273 Covid 19 Vaccine Effectiveness Against The B 1 1 7 And B 1 351 Variants And Severe Covid 19 Disease In Qatar Nature Medicine

Evaluation Of Covid 19 Vaccination Strategies With A Delayed Second Dose Medrxiv

Beyond The First Dose Covid 19 Vaccine Follow Through And Continued Protective Measures Nejm

Public Health Impact Of Delaying Second Dose Of Bnt162b2 Or Mrna 1273 Covid 19 Vaccine Simulation Agent Based Modeling Study The Bmj

Could A Single Dose Vaccine Strategy Be More Beneficial In Covid 19

Interim Findings From First Dose Mass Covid 19 Vaccination Roll Out And Covid 19 Hospital Admissions In Scotland A National Prospective Cohort Study The Lancet
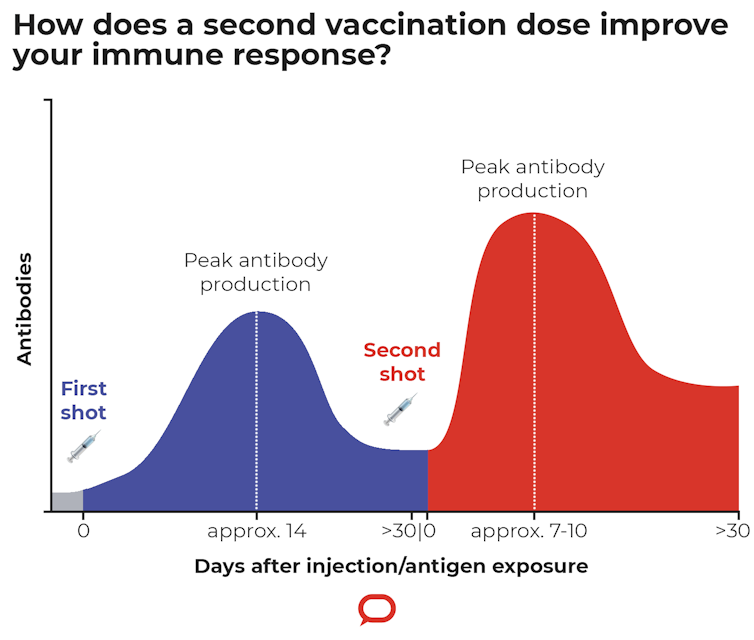
Should I Get My Second Astrazeneca Dose Yes It Almost Doubles Your Protection Against Delta
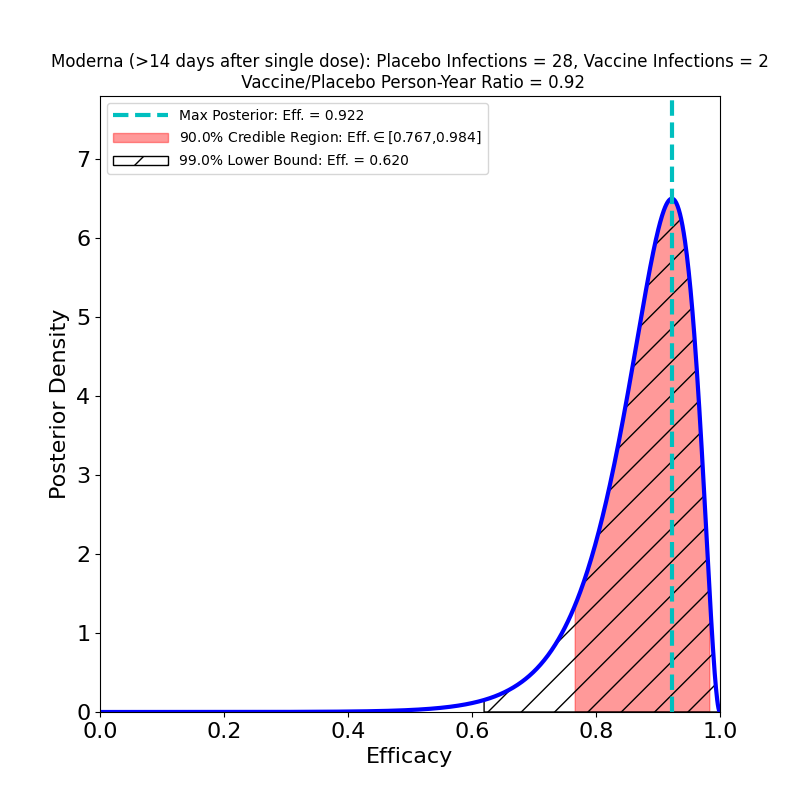
Covid 19 Vaccine Efficacy Page Vaccine Efficacy Comparisons Made Easy
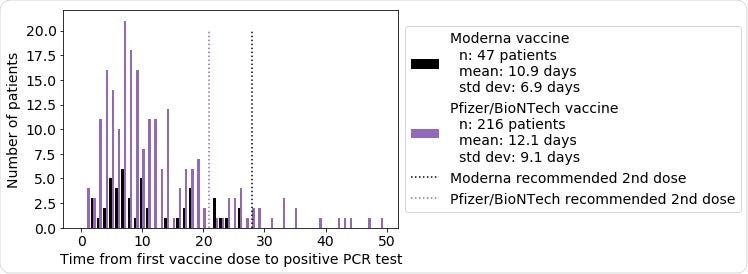
Study Shows Real World Effectiveness Of Moderna And Pfizer Biontech Vaccines

Pfizer Biontech Vaccine Starts Working 10 Days After First Dose Says Fda Financial Times
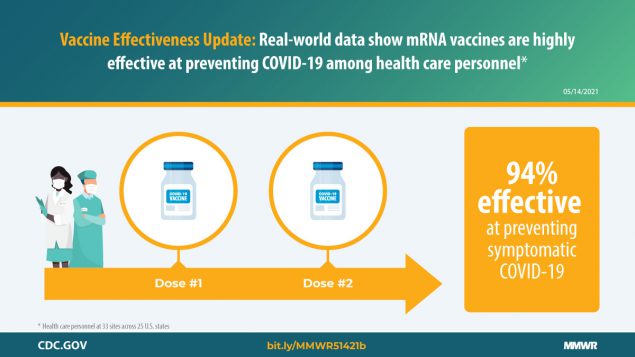
Interim Estimates Of Vaccine Effectiveness Of Pfizer Biontech And Moderna Covid 19 Vaccines Among Health Care Personnel 33 U S Sites January March 2021 Mmwr

Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm

Us Regulator Finds Moderna S Covid 19 Vaccine Highly Effective Financial Times

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet